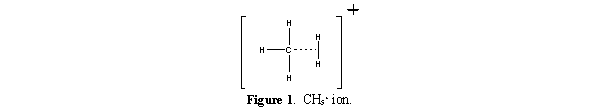
In Chemical Ionization the source is enclosed in a small cell with openings for the electron beam, the reagent gas and the sample. The reagent gas is added to this cell at approximately 10 Pa (0.1 torr) pressure. This is higher than the 10-3 Pa (10-5 torr) pressure typical for a mass spectrometer source. At 10-3 Pa the mean free path between collisions is approximately 2 meters and ion-molecule reactions are unlikely. In the CI source, however, the mean free path between collisions is only 10-4 meters and analyte molecules undergo many collisions with the reagent gas. The reagent gas in the CI source is ionized with an electron beam to produce a cloud of ions. The reagent gas ions in this cloud react and produce adduct ions like CH5+ (Figure 4), which are excellent proton donors.

When analyte molecules (M) are introduced to a source region with this cloud of ions, the reagent gas ions donate a proton to the analyte molecule and produce MH+ ions. The energetics of the proton transfer is controlled by using different reagent gases. The most common reagent gases are methane, isobutane and ammonia. Methane is the strongest proton donor commonly used with a proton affinity (PA) of 5.7 eV. For softer ionization, isobutane (PA 8.5 eV) and ammonia (PA 9.0 eV) are frequently used. Acid base chemistry is frequently used to describe the chemical ionization reactions. The reagent gas must be a strong enough Brønsted acid to transfer a proton to the analyte. Fragmentation is minimized in CI by reducing the amount of excess energy produced by the reaction. Because the adduct ions have little excess energy and are relatively stable, CI is very useful for molecular mass determination. Some typical reactions in a CI source are shown in Figure 5.
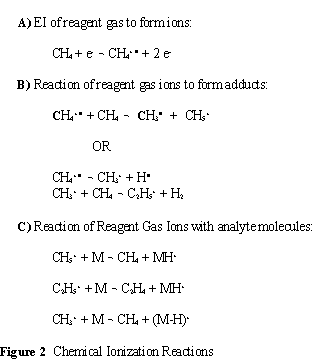
1. Munson, B. Anal. Chem. 1977, 49, 772A-778A.
2. Munson, B.; Field, F. J. Am. Chem. Soc., 1966, 88, 2621-2630.