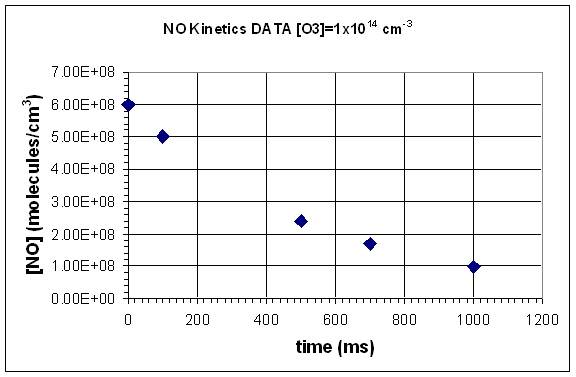
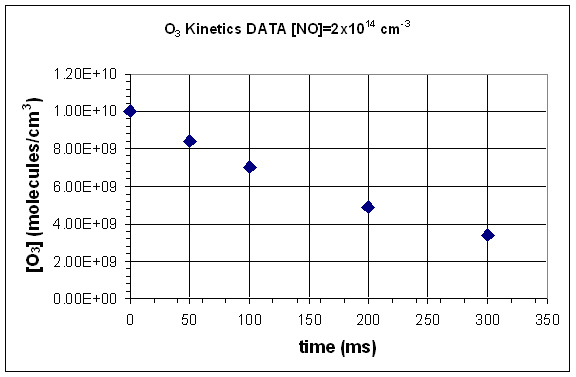
- Graph each data set.
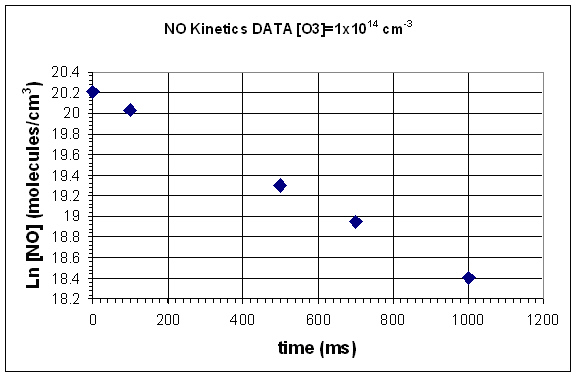
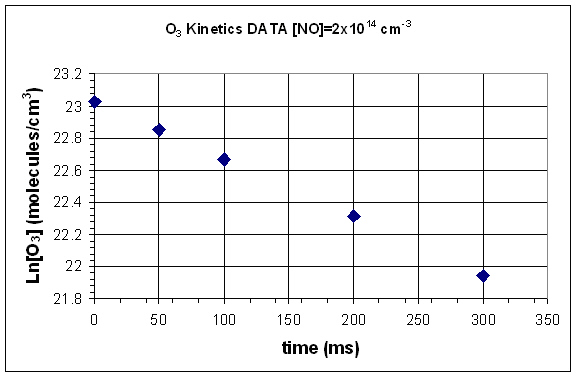
- Graph ln[] vs time.
- Determine the average rate for the reaction, between each data point.
- Use your graph to determine the instentaneous reaction rate at 250 ms.
- Given that the reaction is first order in NO and in O3, determine the rate constant using your calculated rate for each set of data points
- Use the ln[] vs time graph to determine the rate constant.
- What is the overall rate law?
- Convert the units of the rate constant to moles, liters, and seconds.
| T (K) | k(L mole-1 sec-1) |
| 195 | 1.08 * 109 |
| 230 | 2.95 * 109 |
| 260 | 5.42 * 109 |
| 298 | 12.0 * 109 |
| 369 | 35.5 * 109 |
- Graph this data as ln(k) vs 1/T.
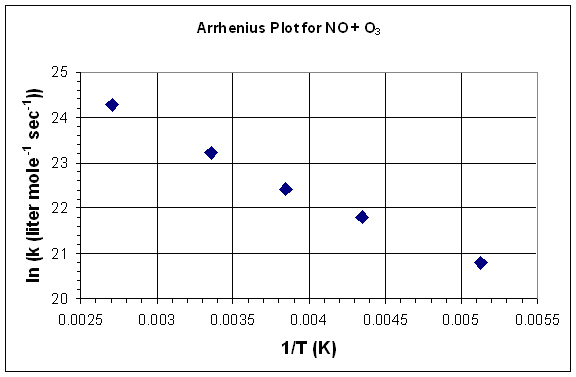
- Determine the activation energy and the preexponential factor from the graph.
- What is the rate constant at 150 K?
- For the above reaction mechanism with several additional steps as shown below:
- Which species is a catalyst?
- Which species is an intermediate?
- How does this catalyst effect the rate of the reaction shown below. Ea = 11.9 kJ for the catalyzed reaction and Ea = 14.0 kJ for the uncatalyzed reaction.
Calculate the change in the reaction rate at 200, 250, and 300 K
O3 + O -> 2 O2 - Draw an energy level diagram for this reaction, with and without catalysis.
- For the catalytic destruction of O3 by Cl Ea = 2.1 kJ, Compare the reaction rate for the uncatalyzed reaction and the Cl catalyzed reaction at 250 K.
Step 1: NO + O3 -> NO2 + O2
Step 2: NO2 + O -> NO + O2
Overall: O3 + O -> 2 O2